Exactly how does a water softener work?
And What Do They Do?
If, like 85 percent of Americans according to the US Geological Survey, you have hard water – which is water loaded with calcium and magnesium – you’ll need a water softener to make it more manageable. Water softeners get rid of the calcium and magnesium in hard water and, among other things, ensure that your appliances don’t succumb to limescale build-up, ultimately leading to costly replacement.
But, in the infamous words of the late Billy Mays, “that’s not all.” Since hard water is, by definition, hard to wash with, your soap won’t lather properly, and this causes all sorts of issues for your health and home. If you have a hard water problem, for instance, you almost certainly also have a dry skin problem. The same soap scum residue that you see on your dishes and silverware is also forming on your skin, clogging your pores just like limescale build-up is clogging your pipes. When you shower or wash your hands, the minerals themselves might also be left on your skin, soaking up the natural oils that keep you looking healthy and vibrant.
In any case, in order to get rid of hard water, you’ll need a water softener.
Now that we’ve gone over why water softeners are important, let’s examine exactly how they work. If you don’t know how water softeners work, you have a significantly increased chance of getting ripped off. You don’t want to buy something that doesn’t work, and – as we’ll see later on in this article – water softener manufacturers make some dubious claims about the efficacy of their products.
In this article, we’ll go over the main mechanisms behind how different types of water softeners remove or manage unwanted contaminants, including:
-
Distillation
-
Reverse osmosis
-
Electromagnetism
-
Ion exchange
-
Template-assisted crystallization

Distillation
The strict dictionary definition of distillation is “the act of purifying a liquid by a process of heating and cooling.” According to the University of Nebraska-Lincoln, distillation is one of the most effective methods for softening hard water, removing up to 99.5 percent of impurities – but it’s also one of the most expensive, making it a poor option for everyday use. Furthermore, because distillation is such a thorough process, it also removes some oxygen from the water. Many people say this causes the water to have a “bland” taste. (If you’ve ever had a glass of distilled water, you get the idea).
How does distillation work?
Using the diagram above from North Dakota State University’s “Treatment Systems for Household Water Supplies: Distillation,” we’ll explain.
-
Water enters the system from the drain.
-
Water is boiled by the heating element, creating steam.
-
Steam forms around the condensing coil and drips into the storage tank.
-
The tap releases distilled water.
Naturally, of course, any impurities such as lead, sodium, chlorine, magnesium, and calcium are left behind in the boiling chamber. Now, you might be thinking, “isn’t it kind of gross to boil all those minerals? Won’t you get sick?”
No, not really. Since the water is so hot, it doesn’t really matter. (Also the impurities are periodically removed through a discharge line).
Within five minutes of exposure, New York’s Department of Health reports that a 99.999% kill of water borne microorganisms can be achieved at 149°F/65°C. Since the boiling point of water isn’t reached until 212°F/100°C, it’s safe to say that distillation kills or inactivates almost all viruses, bacteria, protozoa, and other pathogens.
If you’re at all confused, a shorter, more readable explanation of water distillation might include only a couple points:
-
Water is boiled.
-
Water is cooled while contaminants are left behind.
That’s pretty much it. Activated carbon filtration is also very similar; in fact, in some ways it’s a form of distillation itself. We’ll go over that in a little bit.
For now, let’s look at another method of water softening.
Reverse Osmosis
The strict dictionary definition for reverse osmosis is “a process by which a solvent passes through a porous membrane in the direction opposite to that for natural osmosis when subjected to a hydrostatic pressure greater than the osmotic pressure.” And, while that sounds pretty difficult to understand, the underlying concept is actually really simple. We’ll break it down.
Regular Osmosis
In order to understand reverse osmosis, you first need to understand regular osmosis. Unlike reverse osmosis, regular osmosis is a passive process, so it needs no pressure in order to work.
Essentially a low solute concentration passes through a porous membrane to join a high solute concentration. Here’s a diagram courtesy of the University of Texas:
In the initial state, the water levels are roughly equal. In the final state, the water passes through the semi-permeable membrane to the high solute concentration.
If you’re having trouble understanding this, I guarantee you’re just getting caught up in the jargon. Instead, think about putting a piece of dried fruit in a glass of water (or, better yet, putting one of those expanding sponge dinosaur toys in the bathtub). The fruit (or dinosaur) is highly concentrated. The water is much less concentrated. The water flows into the fruit or dinosaur through its skin (a semi-permeable membrane) and returns it to its original size – or at least pretty close to its original size.
(Caption: Throw one of these guys into a glass of water to see osmosis in action.)
That’s osmosis. Pretty simple, right?
Reverse Osmosis
Now, reverse osmosis is just the opposite. Water softeners’ primary aim is to get rid of unwanted contaminants like magnesium and calcium, and reverse osmosis achieves that by pushing the contaminated water against a filter, thereby keeping the contaminated water on one side of the filter and the decontaminated water on the other side. It’s like if you were to squeeze the dinosaur toy, forcing all of the water out of it – or if you somehow pulled the water out of a grape or banana in order to make some dried fruit.
The Process
Since we know that, in regular osmosis, dilute solution flows through a membrane toward highly concentrated solution, we need pressure in order to reverse this process.
-
Concentrated solution (hard water) is stored in a tank opposite the diluted water.
-
Concentrated solution is pressurized so that it passes through a semi-permeable membrane (filter).
-
Contaminants (magnesium and calcium) are filtered out through a waste drain.
-
Treated water is pumped into the main water line.
And that’s pretty much it.
We’ve looked at two forms of water softening, distillation and reverse osmosis, that remove 99% of contaminants from hard water. Now we’re going to analyze a much more controversial method of water softening:
Electromagnetism Salt-Free Water Softeners
Having water run through a series of magnets in order to change the crystalline structures of magnesium and calcium – and therefore the way they interact with other structures – is a proven phenomenon and technology. For example, Aqua-Flo Inc. has conducted over 25 independent studies proving the effectiveness of their magnetic water conditioning equipment. Also, Jon Eakes, an expert in home renovation and all things fixer-upper for the past 38+ years, says, “Magnetic and electronic water conditioning can be legitimate if the mineral composition of your water is appropriate for their use [you have a hard water problem] and the units are sized for your water flow.”
Bottom line: Magnetic water conditioners work, but there are plenty of conditioners on the market that, because of their size, don’t really work the way that they should.
Still, the underlying controversy lies not in the existence of electromagnetism, but the semantics over whether or not electromagnets really “soften” water or “condition” it.
As we’ve seen in the above two examples with distillation and reverse osmosis, magnesium and calcium ions are actually removed from the water, leaving a largely contaminant-free solution behind. Electromagnetic water softeners might not remove the calcium and magnesium from the water itself, but that doesn’t mean that they’re completely ineffective as water softeners.
If calcium is left in suspension in water, eliminating limescale build-up, then that water could be said to be “soft water.” However, the actual chemical make-up of the water will remain “hard.” Let’s examine why that might not be a bad thing.
The Health Benefits of Magnesium and Calcium
According to Harvard Medical School, magnesium is important because:
-
It’s involved in more than 300 chemical reactions in the body.
-
Muscles need magnesium in order to contract.
-
Nerves need it to send and receive messages.
-
Keeps your heart beat steady.
-
Keeps your immune system strong.
And, according to the National Osteoporosis Foundation, calcium is important because:
-
It builds healthy, strong bones.
-
It enables our blood to clot.
-
It lets our muscles contract (repeat).
-
We lose calcium every day through our sweat, nails, hair, urine, and feces – and our bodies don’t produce it on its own. We need to get calcium through our diet.
The list goes on and on: magnesium and calcium are very, very good for you – no matter how you look at it. While hard water might be causing a nuisance on your appliances (and skin, as we mentioned above), electromagnetic water conditioners affect the negative aspects of hard water while keeping the positives.
There’s another type of water softening, ion exchange, which is involved in salt-based water softeners. Instead of boiling the magnesium and calcium out of the water or filtering it out, these work a little bit differently.
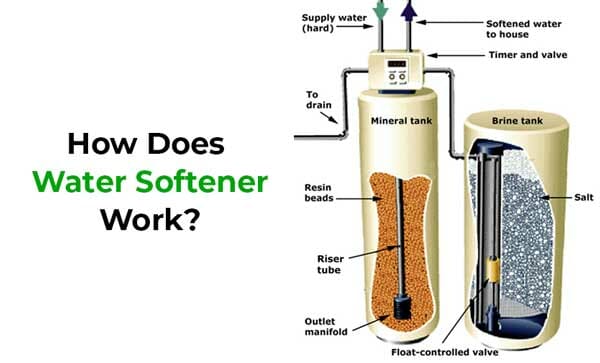
How Does Ion Exchange Works
Cations are positively charged ions dissolved in water, just like the positively charged magnesium and calcium in hard water. Cation exchange, then, consists of replacing hardness ions with nonhardness ions, like sodium.
This diagram, courtesy of North Dakota State University, lays out the basics of ion exchange. On the left is the actual process of ion exchange and on the right is the process of regeneration.
The Process of Ion Exchange (The Diagram on the Left)
-
Hard water is pumped into a storage container full of zeolites, which are negatively-charged little beads of sand meant to attract and bind magnesium and calcium ions. Before hard water is pumped into the mineral tank, the zeolites are bound with sodium.
-
Magnesium and calcium binds to the zeolite, effectively knocking the sodium off the beads.
-
Treated soft water is pumped into the house. It should be noted, though, that this water is full of sodium.
The Process of Regeneration (The Diagram on the Right)
-
Eventually all of the zeolites are fully saturated with magnesium and calcium. You don’t have to manually remove these zeolites from the mineral tank and buy some more just because you ran out. However, since they’re already covered with magnesium and calcium, they aren’t going to be effective.
-
So, brine solution (which is highly concentrated salt solution) is pumped into the mineral tank.
-
The brine flushes out the magnesium and calcium. (At this point, you might be thinking, “How? If the magnesium and calcium only knock the sodium off of the zeolites because they have a stronger positive charge than the sodium, then how can the sodium knock off the magnesium and calcium just because they’re pumped in the opposite direction? The answer: the brine is so fully saturated with sodium that it’s strong enough to knock off the magnesium and calcium.
-
The hard water is flushed out of the system.
Pretty fascinating, right? Who knew such complicated (but ingenious) methods existed for such a mundane process as water softening? It makes you wonder how anyone came up with all of this – but that’s an article for another day.
The Biggest Issue with Salt-Based Ion Exchange Water Filters
Remember how, in the section on electromagnetic water softeners, we reviewed the health benefits of magnesium and calcium? They help build strong bones, keep your heart beating, and are altogether involved in a long list of important processes.
On the other hand, sodium, well, isn’t.
According to the Center for Disease Control, the vast majority (about 9 in 10) Americans eat way too much sodium. They should be eating about 2300mg per day, at most, but many are consuming roughly 1100mg more than that, at 3400mg per day.
Conversely, according to the National Institutes of Health, there are sections of the American population that aren’t getting enough calcium in their diets, including “females aged 4 years and older—particularly adolescent girls—and males aged 9 to 18 years and older than 51 years.”
Your children might fall into those categories, and chances are if you’re an American your doctor has recommended a low-sodium diet.
Do you really want to replace the healthy calcium and magnesium in your water with the unhealthy sodium? The choice, of course, is yours, but there are better ways…
How Does Template Assisted Crystallization Work
Just like electromagnetic water softeners, template-assisted crystallization changes the way that hard water reacts with surfaces, keeping the magnesium and calcium suspended for more than 72 hours, so that you can enjoy the benefits of hard water with none of the negatives.
You can think of template assisted crystallization as a mix between the electromagnetic water softener and the ion exchange water softener – without any added salt.
Essentially TAC (template assisted crystallization) uses small polymeric beads – just like zeolites – to convert dissolved hardness into microscopic crystals, which remain suspended in the water.
If you’ve been paying attention for this long, then it isn’t very difficult to understand.
Recap
Distillation works by boiling and cooling untreated water, leaving the contaminants behind in a separate tank, where they are then flushed out. The result: contaminant-free water with no viruses, bacteria, or other pathogens. The problem: it’s really, really expensive. Probably way too expensive for the average home. Also, since it’s so effective, it removes some oxygen from the water, leaving the water bland.
Reverse osmosis is the opposite of osmosis. Untreated (highly concentrated) solution is pressurized so that the water passes through a semi-permeable membrane. While this gets rid of your hard water problem, it also gets rid of the healthy magnesium and sodium in your water.
Electromagnetism is highly controversial since it changes the crystalline structures of magnesium and calcium so that they remain suspended in your water. This prevents limescale build-up. The only problem, though, is that a lot of the electromagnetic water softeners on the market don’t work like they probably should – and that’s because the most effective electromagnetic water softeners are usually industrial-sized.
Ion exchange water softeners replace magnesium and calcium ions with sodium ions. Magnesium and calcium are healthy; sodium isn’t. These water softeners are also a pain to manage, requiring you to periodically refill the brine tank and clean out the mineral tank. You might get rid of your hard water problem, but you’ll do it at the cost of your health (and the environment’s health).
TAC is relatively new to the water treatment industry, but it’s a credible alternative to ion exchange and the far less effective electromagnetic water heaters. It’s almost like a hybrid between electromagnetic water softeners and ion exchange softeners.
If you’re interested in buying a template-assisted crystallization softener, or if you have any more questions about the science of water softeners, contact us at 866-455-9989. We’ll be happy to help.